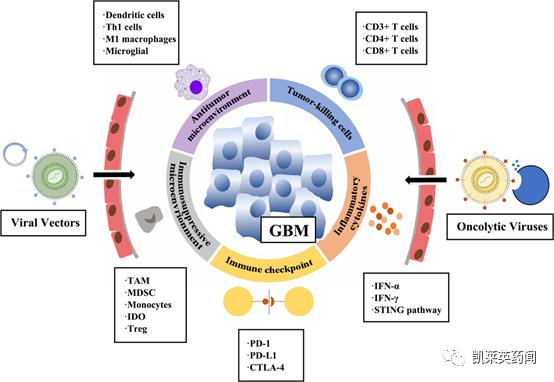
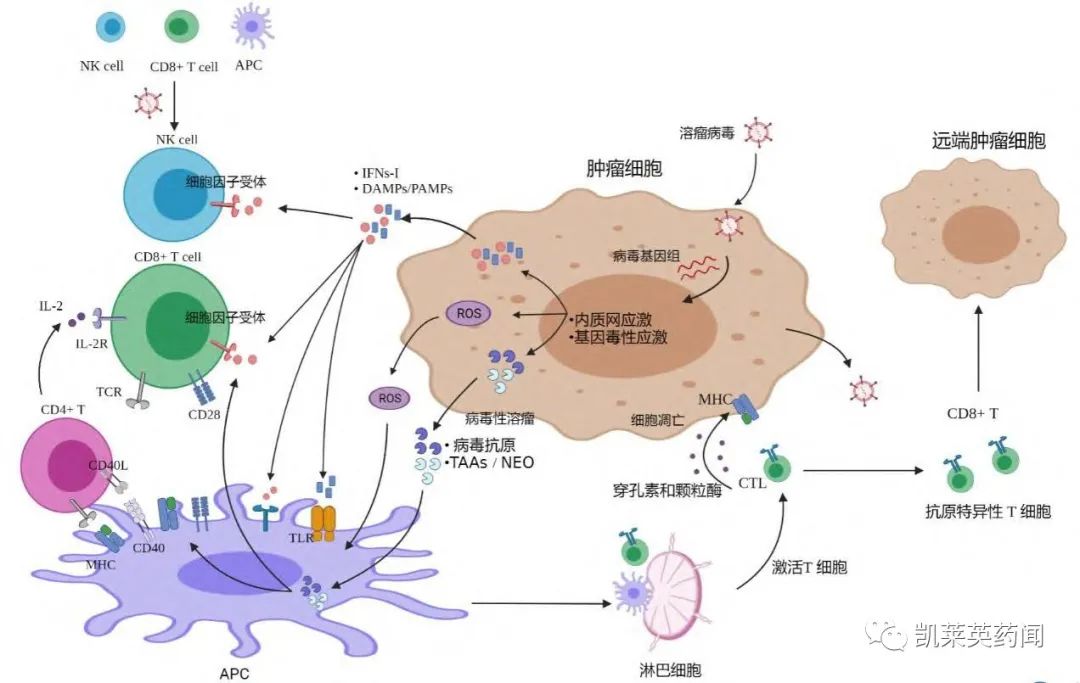
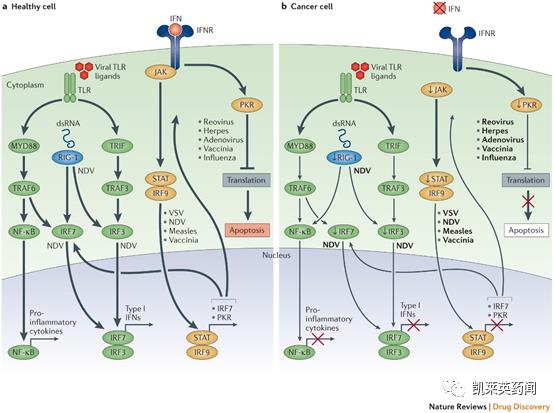
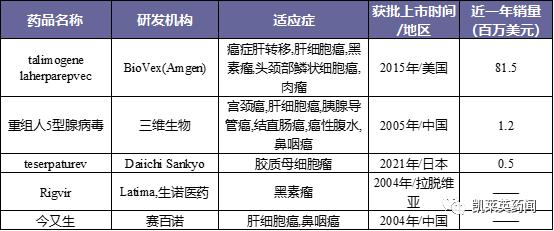
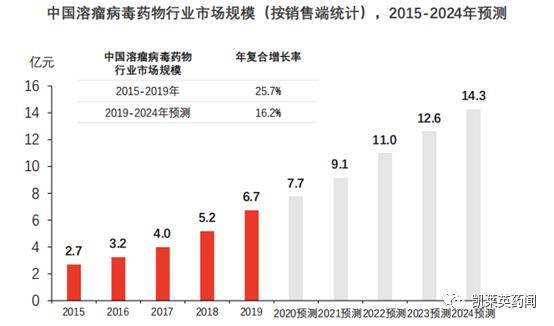
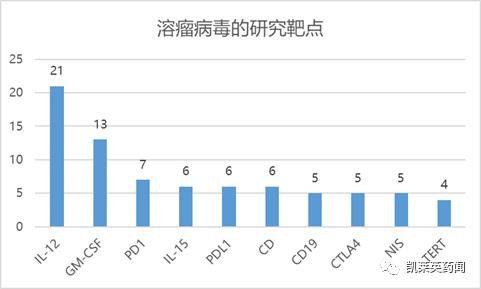
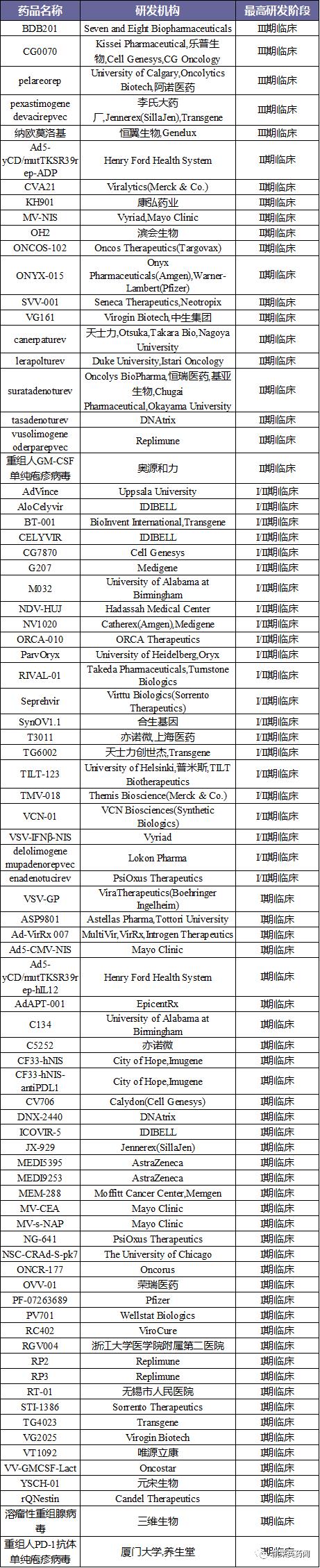
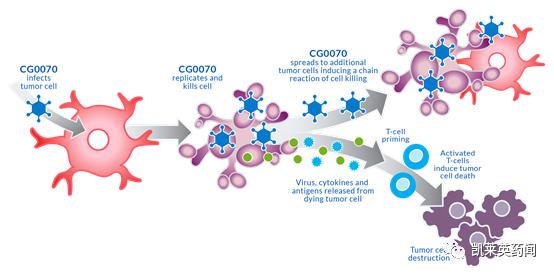
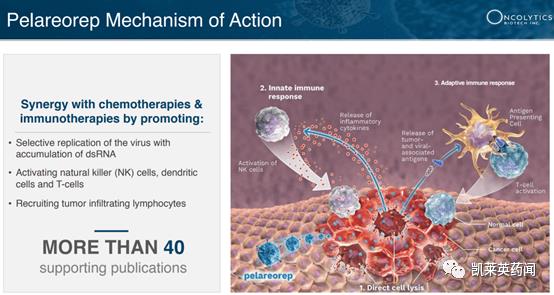
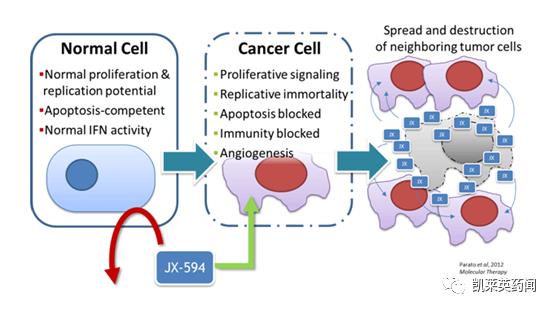
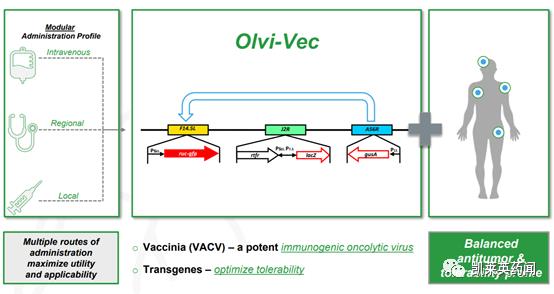
Foreword
For a long time, the United States has instrumentalized and weaponized democracy, carried out anti-democracy in the name of democracy, incited separatism and confrontation, and interfered in other countries’ internal affairs, resulting in disastrous consequences.
The National Endowment for Democracy (NED), as one of the main forces of the "pawns", "white gloves" and "democratic crusaders" of the American government, subverts the legitimate governments of other countries and cultivates pro-American puppet forces under the guise of "promoting democracy", leaving a trail of bad deeds all over the world, causing strong dissatisfaction in the international community.
In today’s world, peace and development are the themes of the times, and the trend of democratization of international relations is unstoppable. Any attempt to interfere in other countries’ internal affairs in the name of democracy is unpopular and doomed to failure.
I. Organizational structure of the National Endowment for Democracy in the United States
After World War II, the United States opened up a hidden front to compete with the Soviet Union through intelligence agencies such as the Central Intelligence Agency. In the 1960s, the United States gradually realized that it was not enough to "promote democracy" by secret means, and it was urgent to establish a "public — Private institutions "provide public funding. In 1983, the National Foundation for Democracy (NED) was established with the color of a cross-party and non-profit organization under the impetus of the then US President and others.
In name, NED is a "non-governmental organization" that "supports democracy in other countries". In fact, it relies on the continuous financial support of the White House and the US Congress. According to the orders of the US government, it manipulates and directs many non-governmental organizations around the world to export American values to target countries and regions, carry out subversion, infiltration and sabotage, incite so-called "democratic movements", etc. In essence, it is a "white glove" of the US government to serve the strategic interests of the United States.
Allen Weinstein, the founder of the foundation, said bluntly when interviewed by Washington post in 1991: "Many things we are doing now are what the CIA did 25 years ago." NED is therefore called "the second CIA" internationally.
NED has four "core recipients": the National Democratic Institute and the International Republican Institute, which are mainly responsible for fostering local political groups; The American center for international labor solidarity is mainly responsible for promoting trade union organizations and workers’ movements; The Center for International Private Enterprise is mainly responsible for wooing private enterprises. Through these four organizations, NED has become the "black hand" behind the scenes of separatist riots, color revolutions, political crises, lies and rumors, and value infiltration around the world, and the evil facts are countless.
二、策动颜色革命,意图颠覆目标国政权
历史上,苏联解体、格鲁吉亚“玫瑰革命”、乌克兰“橙色革命”以及“阿拉伯之春”等美国煽动和策划的“颜色革命”背后均可见NED的身影。
1. NED针对“敌对”国家策动“颜色革命”。基金会早期文件显示,早在上世纪80年代末期NED就主要在东欧活动,颠覆政权。
◆ 1989年8月27日,《华盛顿邮报》发表题为《我们是如何帮助团结工会取胜的》的报道,指出NED向波兰团结工会提供资金支持,帮助他们推翻当时的政府,开启了东欧剧变的序幕。
◆ 2000年10月,NED资助、策动塞尔维亚“天鹅绒革命”,颠覆了米洛舍维奇政府。1999年和2000年,NED分别资助塞尔维亚反对派1000万美元和3100万美元,使其迅速壮大。NED还协助秘密训练了一批大学生,将他们交由一个名为奥帕尔的学生团体领导,随后策划了暴乱。《华盛顿邮报》在对塞尔维亚“天鹅绒革命”的事后剖析中写道,美国资助的顾问在反塞尔维亚运动的几乎每一个方面,都在幕后发挥了关键作用。他们追踪民意调查,训练数千名反对派活动人士,并帮助组织了至关重要的平行计票。
◆ In 2003, the "Rose Revolution" broke out in Georgia, and then President Shevardnadze was forced to step down. NED planned to participate in the color revolution from "selecting" the leader of the opposition, to training personnel for the opposition, to providing huge funds. After the success of the "Revolution", NED continued to "generously distribute money". In 2004 alone, it provided nearly 540,000 US dollars to 12 NGOs in Georgia.
◆ At the end of 2004, during the "Orange Revolution" in Ukraine, the United States provided $65 million to the Ukrainian opposition through organizations such as NED. In 2013, a large-scale anti-government demonstration broke out in Ukraine. NED funded as many as 65 non-governmental organizations in Ukraine and even provided a large amount of funds to "pay wages" for everyone who participated in the protests. The Russian News Agency published an article saying that NED had invested 14 million US dollars in Ukrainian projects, which promoted the big demonstrations in 2014 and overthrew the Yanukovych government at that time.
2. NED is an important behind-the-scenes player of the "Arab Spring" color revolution. In Egypt, Yemen, Jordan, Algeria, Syria, Libya and other countries, by supporting so-called feminist, press freedom and human rights activities, Ned provided financial support to pro-American individuals and groups, exported all kinds of anti-government ideas and incited the color revolution, which led to the Arab world being mired in war, social unrest and economic recession.
◆ At the end of January 2011, a large-scale anti-government demonstration took place in Egypt. On February 11th, President Mubarak resigned. According to materials such as American diplomatic cables obtained by Wikileaks, NED played an important role in organizing and manipulating anti-government demonstrations in Egypt. Through NGOs such as "National Change Movement" and "April 6th Youth Movement", the Foundation provided funds, training and other support for demonstrations. The name and slogan of "National Change Movement" are exactly the same as those of other national anti-government organizations trained by NED.
◆ In Libya, NED funded the founders of the anti-government organization "Libya Humanitarian and Political Development Forum", "Transparent Libya", and the sponsors of the website "Libya News" who fled to London. These organizations were active in the civil war in Libya in 2011.
◆ In Yemen, NED funded and cooperated closely with non-governmental organizations such as "Women Journalists Without Chains" and played an important role in the anti-government protests and demonstrations in Yemen in 2011. Tawaku Kaman, the founder of "Women Journalists Without Chains", organized and led a student rally against the Saleh government.
◆ In Algeria, many organizations participating in the "Arab Spring" protests were funded by NED. NED’s annual report revealed that the Algerian League for the Defence of Human Rights had received funding from the United States in 2003, 2005, 2006 and 2010. The national autonomous trade union of public administrators is closely related to the American Center for International Labor Solidarity under NED.
3. instigated Bolivia’s "color revolution" and forced President Morales to resign and go into exile. Morales left-wing regime has been in power for nearly 14 years. The political situation has been stable for a long time, the economic growth rate has led South America, the poverty rate has continued to decline, the people’s livelihood has improved significantly, and the contradiction between Turkey and Belarus has obviously eased. The left-wing regime of Morales won the general election, but was forced to fall by the "street movement" and the military police, and NED played multiple roles in it. First, the long-term layout cultivates the power of "falling down". From 2013 to 2018, NED and USAID provided $70 million to the Bolivian opposition in various ways, subsidizing "anti-Mo" forces such as Bolivian white elites and former right-wing politicians, weaving "anti-Mo" nets covering universities, think tanks and civic organizations, and even wooing and fostering "Indian columns" among indigenous people. Many prominent opposition figures have received the above-mentioned financial support or have close contacts with the United States. The second is to fabricate the topic of "election fraud" to carry out brainwashing actions. Since 2018, NED has invested US$ 45,000 and US$ 42,000 respectively through the Media Fund and the Fiddes News Agency Foundation (FIDES) to guide the right-wing media in Bolivia to dig up the government’s corruption and abuse of power.Black material ",on the grounds that Morales was re-elected, labeled Morales as a" dictator ". Through the "Millennium Fund", 45,000 US dollars were invested to help Bolivian universities, chambers of commerce, non-governmental organizations and other key topics such as "election justice" and "judicial transparency" to create psychological expectations of Mozambique’s "election fraud". The third is to support "street sports" behind the scenes. On October 29, 2019, after the election results were announced, opposition leaders such as Mesa organized a "peaceful demonstration" on the streets, demanding re-election and distributing cash to the demonstrators on the spot. Camacho, the leader of the opposition, later became the key propaganda target of the right-wing media supported by NED, which incited the national strike and became the "spokesperson of the United States". NED also contributed $200,000 through its core transferee organization, the International Republican Institute, to improve the mobilization and organization ability of opposition political parties and provide guidance for "street movement".
Third, collude with local political groups and interfere in the political agenda of other countries.
NED has infiltrated the target country for a long time, cultivated local anti-government forces, constantly intensified social contradictions, and extended its black hand to other countries’ internal affairs.
1. Interfering in Hong Kong elections and interfering in China’s internal affairs. NED mainly contacts with opposition political parties and organizations in Hong Kong through its affiliated American Democratic Association for International Affairs. Since 1997, the organization has issued 18 evaluation reports in an attempt to influence the "democratization process in Hong Kong". In 2002, the American Democratic Association for International Affairs set up an office in Hong Kong. In 2003, it funded the opposition to instigate the "July 1 March" and prevented "23 pieces of legislation"; In 2004, sponsored Hong Kong opposition political groups to participate in workshops and seminars, provided personal consultation to the leaders of political groups, and taught them election skills; In 2005, the so-called "Young Political Leaders Program" was launched to train emerging political groups to confront the government. In 2006, funded the "Hong Kong Transition Research Plan"; In 2007, its activities in Hong Kong were divided into four projects: "Commitment to Democratization in Hong Kong" series reports, opinion polls, youth public participation and women’s political participation; In 2008, organize a student summit; In 2010, he planned a "five-district referendum" with opposition legislators; In 2012, funded the University of Hong Kong to open the website of "Hong Kong people talk about universal suffrage", recruited university interns in Hong Kong, and sponsored youth summits; In 2014,Instruct and fund Hong Kong opposition and radical young cadres to plan illegal "Occupy Central" actions.
NED official website shows that in 2020, the capital involved in Hong Kong will be 2 million US dollars, and there will be 11 projects, among which disrupting the Legislative Council election will be an important task, with the focus on the project of "strengthening citizens’ supervision over elections", providing technical and financial assistance to newly established chaotic groups in Hong Kong and encouraging them to disrupt the Legislative Council election by supervising elections and competing for voting rights; The project "Broaden the horizon of citizens’ political participation" collects and disseminates the results of public opinion surveys on democratic development, and induces young people in Hong Kong to share their experiences of political participation through the Internet; Before the Legislative Council election, the project of "Supporting the Unity of Student Movers" will promote the interconnection among Hong Kong student groups, guide and train their ability to promote "democratic change" and publicize internationally, and participate in disrupting the election order; The project "Building Regional Unity and Empowering Hong Kong Democratic Movement" strengthens Hong Kong’s "democratic movement" through the network, cultivates the next generation of Hong Kong’s "activity leaders" and lays a "democratic movement" network in Asia.
2. Interfering in Russian elections and threatening Russian constitutional laws, national defense and national security. According to the Russian General Prosecutor’s Office, from 2013 to 2014, the Foundation allocated 5.2 million US dollars to local organizations. In July 2015, Russia listed NED as an "unwelcome organization". The official Russian statement pointed out that NED "participated in boycotting Russian election results, organizing political demonstrations, trying to influence the decisions of Russian government agencies and discrediting the Russian armed forces".
3. Disrupt the political situation in Belarus. In 2006, 2010 and 2020, the United States instigated the "color revolution" against the Belarusian regime three times, and NED has always played an important role in it. In 2020, the total amount of projects in Belarus reached 2.35 million US dollars, among which NED launched the project of "Promoting Free and Fair Elections" on the grounds of promoting the political process, with a total amount of 80,000 US dollars. The content of the project was to carry out a comprehensive publicity campaign to educate citizens about election rights and independent election supervision before the presidential election; During the election campaign, activists were educated and trained on voting issues, observers were deployed to monitor the voting process, and the monitoring results were disseminated through various media.
On August 9, 2020, Lukashenko, the current president of Belarus, was elected for the sixth time with 80.1% of the votes. The Belarusian opposition questioned election fraud, which led to large-scale protests in cities such as Minsk, the capital, for several days, and riots in some areas. During the riot, NED had frequent activities. On May 17th, 2021, "Russia Today" TV station released a video conference between NED executives and Belarusian opposition figures. In the video conference, Carl Gershman, then chairman of NED, personally admitted that NED has been working all over Belarus for a long time and participated in the "citizen movement" in Vitebsk and Gomel in the east. NED supports opposition leader Tikhanov Skaya, and cooperates with Tikhanov Skaya’s team through its core transferee organization to help them carry out related activities.
Dmitry Egorchenkov, an expert on Russian international relations, summed up NED’s activities in Belarus, saying that NED provided financial support to many "independent media", usually with a small amount for a single media, but with many recipients. According to the data of NED official website, from 2016 to 2020, there were 119 "freedom of information" projects funded by NED in Belarus, with an average funding of about 50,000 US dollars for each project, ranking first in all categories for five consecutive years.
4. Intervention in Mongolian parliamentary elections. In 1996, Mongolia held parliamentary elections, and the American Institute of International Republics under NED was deeply involved. In its annual report in 1996, the American Institute of International Republics disclosed that since 1992, the organization has trained the opposition parties in Mongolia in party member’s recruitment, organizational construction and election campaign. Under its instigation, Mongolia’s scattered "democratic" forces first integrated into two political parties, and then formed a unified opposition Coalition in early 1996, winning 50 of the 70 seats in the Mongolian parliament. According to several annual reports of NED, from 1992 to 1996, the foundation allocated more than 480,000 US dollars to the American Institute of International Republics. In 1996 alone, nearly 160,000 US dollars were used to support the Mongolian opposition alliance to win the election.
5. "Supervise" Kyrgyzstan’s elections and constitutional referendum. From 2013 to 2020, NED directly allocated more than $13 million to Kyrgyz media and various non-governmental organizations. In 2020, the Foundation will provide more than $2 million for various "destructive news" projects in Kyrgyzstan. Among them, the Foundation allocated $300,000 to "Krupp Website" to "supervise" the referendum on constitutional amendment and local council elections in Kyrgyzstan. In January 2021, during the presidential election in Kyrgyzstan, the website recruited 1,500 "observers", and in April, during the local Council election and constitutional referendum, it recruited 3,000 "observers".
6. Incite protests and demonstrations in Thailand. There were many street protests and demonstrations in Thailand in 2020. Organizations such as "Thai Human Rights Lawyers" funded by NED have publicly supported and incited street protest movements. Thailand’s Bangkok Post once revealed that "Thai human rights lawyers" accepted NED funds. According to Thailand’s "National News", NED also provided funds for media platforms such as Prachatai, a Thai online media, and NGOs such as iLaw, an Internet legal institution. Through these platforms and organizations, it asked the Thai government to amend the Constitution, thereby intervening in Thailand’s internal affairs.
7. Encourage the Nicaraguan opposition to seize power by violence. After its establishment in 1983, NED’s first projects included supporting pro-American political forces in Nicaragua, a Central American country. From 1984 to 1988, the foundation provided about $2 million to the Nicaraguan opposition, helping the opposition violeta Chamorro to be elected president in 1990. Today, NED still sends funds to Nicaraguan opposition and right-wing media through the Chamorro Reconciliation and Democracy Foundation, which was established after Chamorro stepped down. According to public information, from 2016 to 2019, NED provided at least $4.4 million to opposition groups (including media organizations) in Nicaragua. These forces played a key role in the violent coup attempt in Nicaragua in 2018, and they even called on opposition supporters to attack the government and assassinate the president.
?
9. Long-term interference in Venezuela’s internal affairs. After Hugo Chá vez, an "anti-American fighter", was elected president in 1999, NED stepped up its black-box operation and continued to provide funds to the Venezuelan opposition to organize centralized training in the form of inviting personnel to visit the United States. Since 1999, NED has been carrying out activities in Venezuela through the office of USAID and the core transferee organizations located in the US Embassy, and has contacted dozens of institutions, opposition political parties and organizations in Venezuela under the pretext of promoting democracy, resolving conflicts and strengthening civic activities, and provided them with funds for activities. NED’s funds for intervention in Venezuela have increased year after year, reaching $257,800 in 1999, ranking first among Latin American countries; In 2000, it soared to $877,400; In 2002, the Bureau of Democracy, Human Rights and Labor in the State Council, USA, specially allocated 1 million dollars to support the projects of the National Foundation for Democracy in the United States. In 2019, the total amount of NED projects in Venezuela reached 2.66 million US dollars. Among them, NED launched the project of "Strengthening Outreach, Communication and Organizational Ability" on the grounds of "Promoting the Political Process", with a project amount of 90,000 US dollars, which aims to provide training and support for local activists, strengthen the communication ability of participants, and build and strengthen the "Venezuela’s domestic"Civil society "network, set up a communication group to spread" democracy "information throughout the country.
In October 2005, five Venezuelan "student leaders", including Guaido, arrived in Belgrade, Serbia to receive "uprising" training funded by NED. After the training, Guaido and others returned to China to promote extreme right-wing ideas, with a view to influencing young people in Venezuela, and planned a series of street violent political activities. Subsequently, Guaido went to study in the United States and has been active in relevant political groups in the United States with the support of NED. After Guaido claimed to be the "interim president", his Wikipedia data grew from scratch in a short period of time, and it was also revised 37 times by the organization under NED to cooperate with Guaido’s "legitimate ruling propaganda". In November 2021, the TV station "Russia Today" issued a document saying that a series of recent internal documents in the United States revealed how the United States interfered in the election process of the Committee. The document shows that the US intelligence department weaponized social media, helped the right-wing opposition political forces, and assisted its members in running for Congress, laying the foundation for Guaido’s self-styled "interim president".
The four core grantees of the Foundation all have extensive activities, established close ties with the opposition parties in the country, and helped to train existing or newly established opposition parties in terms of organization, management and publicity. Provide a number of financial assistance to the largest opposition trade union to promote the latter to launch a protest March against Chavez. On January 10, 2019, Venezuelan President Maduro was sworn in. The United States and other countries refused to recognize his new term, and instigated Juan Guaido, chairman of the National Congress (speaker) and leader of the opposition party, to set up another hill and openly confront Maduro. Guaido then called himself "interim president" and demanded re-election, and then the country fell into riots. Facts have proved that the chaos of the Communist Party of China is obviously the result of the "color revolution" instigated by American support agents, including many years of NED’s management of the opposition. In March 2019, Venezuelan Foreign Minister Jorge Arreaza accused many organizations of sabotaging Venezuela for more than 20 years, with the support of NED, trying to overthrow the Venezuelan government.
10. Organize a violent coup to "change the sky" in Haiti. In 2001, the American Institute of International Republics was deeply involved in the violent coup in Haiti and overthrew the then democratically elected President Aristide. In February, 2001, Stanley Lucas, the head of the Haiti project of the American Institute of International Republics, made a speech on Haiti Radio, publicly throwing out three strategies to let Aristide step down. Roger noriega, then Assistant Secretary of State of the United States, not only cooperated with the International Republican Institute of the United States to provide funds for the Haitian opposition, but also acquiesced in the separatist strategy of the opposition in mediating the political crisis in Haiti. The American Institute of International Republicanism advertised as "promoting democracy around the world", but in fact, it has been in close contact with the Haitian opposition for a long time and carried out subversive actions.
11. Foster opposition leaders to participate in Uganda’s general election. In January 2021, Uganda held a presidential election. Robert Chiagulani Sentamu, the presidential candidate of the opposition National Unity Platform Party, ranked second with 34.83% of the votes. Sentamu grew up in a slum, used to be a pop singer, and then entered politics. Some analysts believe that the reason why Sentamu has such a strong appeal is closely related to the support of the United States. According to online media, in 2018, at the invitation of NED, he received relevant training on subversion of political power in the United States in the name of seeking medical treatment. In addition, NED also provided him with funds and appointed staff officers to support his participation in Uganda’s general election.
Fourth, funding separatist forces and undermining the stability of the target country
China has always been one of the key targets of NED’s infiltration and subversion activities. NED invests huge sums of money in anti-China projects every year in an attempt to incite "Xinjiang independence", "Hong Kong independence" and "Tibet independence". According to the published data of NED official website in 2020, NED provided more than $10 million to 69 projects related to China in a year, in an attempt to promote all kinds of activities that endanger the political and social stability of China.
1. NED是诸多“疆独”组织的主要资金来源。该基金会称,2004年到2020年,它向各种“维吾尔组织”提供了875.83万美元资金。仅2020年就向各类“疆独”势力提供约124万美元资金,其中大部分流向“世界维吾尔代表大会”等“疆独”组织。基金会时任总裁格什曼曾公开妄称“中国新疆问题的解决之道是在中国进行另一场颜色革命,中国发生政权更迭,成为一个联邦共和国”。2019年6月,格什曼在该基金会的“民主奖”活动上,公开发表支持“东突”的言论,为“疆独”势力张目。其后,他还呼吁全球关注所谓新疆人权问题,妄图建立一个国际联盟专司新疆人权事务并对中国进行制裁。
美国“灰色地带”网站揭露,多年来,NED直接资助“世维会”和“美国维吾尔协会”数百万美元,协助其与美西方国家政府、国会合作,不断升级与中国的敌对状态。“美国维吾尔协会”主席库扎特·阿尔泰公开称“我能想象到最正常的事情就是每一天都开展反华活动”。“灰色地带”调查报告显示,2020年在美国新冠肺炎疫情大流行期间,“美国维吾尔协会”及其骨干极力攀附美极右翼政治势力,鼓噪“中国病毒”,煽动反亚裔仇恨情绪。
?炮制报告抹黑中国涉维吾尔族政策;“维护和倡导维吾尔人的人权”项目和“增强妇女和青年在宣传和公民参与方面的能力”项目,延续2019年涉疆工作。
2.NED与“藏独”势力保持密切接触。自2010年NED时任主席格什曼向达赖颁发“民主服务奖章”起,双方开始接触。2016年格什曼出席达赖的“希望与民主”活动,2020年庆祝达赖85岁生日,声援达赖“藏独”活动。2018年11月13日,NED在美操办涉藏问题研讨会,邀请时任伪“西藏流亡政府”“首席噶伦”洛桑孙根参会。洛桑孙根在会上大放厥词,诬称“中国的援助计划最终目的是殖民”,“国际社会需要从西藏的经历中吸取教训,认识到中国隐藏在‘一带一路’计划下的野心”。2021年6月16日,在NED组织下,“藏人行政中央”新“司政”边巴次仁公开接受美《华盛顿邮报》记者、专栏作家乔什·罗金采访,鼓吹“致力于为恢复停滞不前的藏中和谈找到一个持久、互利和非暴力的解决方案,新一届的噶厦将加强国家关系和宣传工作”。
NED涉藏项目聚焦壮大“藏独”势力,推动西藏问题国际化。2019年,涉藏资金60万美元,重点项目包括:“加强西藏运动和领导能力培训”项目,加强“藏独”分子开展西藏社会运动,游说和施压国际社会干预西藏事务;“加强对西藏地区民主和人权的国际支持”项目,培育本土“藏独”势力,强化境内外勾连,策划和实施西藏社会运动;“赋予新一代西藏领导人权力”项目,培植新一代“西藏社运领袖”;“为对话和谈判创造条件”项目,通过所谓学术研究为“藏独”张目。2020年,资金100万美元,重点项目包括:“西藏时报”项目,发行藏文报纸,运维藏文网站,为伪“西藏流亡政府”和“藏独”组织活动提供平台;“国际声援西藏人权运动”项目,收集西藏人权问题有关证据,在联合国场合抹黑中国政府治藏政策;“增强对班禅喇嘛的认识”项目,混淆国际社会对第十一世班禅喇嘛的认识和支持,抹黑中国宗教自由政策;“加强西藏监测信息网络建设”项目,提高对西藏人权监测、记录,炮制涉藏负面报告;“促进藏族选民的知情投票”项目,培养藏人参与“流亡政府”选举决策的能力。
3.全面支持“港独”。NED长期在香港开展“劳工权利”“政治改革”“人权监察”相关项目,香港街头示威活动都能找到NED的影子。香港舆情分析机构“正思香港”研究NED官网发现,从1994年起,该基金会即开始资助“香港人权监察”“香港职工盟”等各种香港反对派组织、学运组织和媒体,操控它们开展各种示威抗议活动。据重庆大学经略研究院研究员杜佳统计,自1994年起,NED每年都资助香港项目,到2018年总投入超过1000万美元。
From 2003 to now, NED has organized, planned, directed and delivered funds behind the scenes in many large-scale street movements in Hong Kong, such as illegal "Occupy Central" and "anti-amendment" violent demonstrations. In the "case-amending storm" in Hong Kong in 2019, NED went from behind the scenes to the front of the stage, and directly contacted the backbone of the anti-China chaos in Hong Kong, providing subsidies and training to those involved in the riots: In May 2019, Hong Kong Democratic Party founding chairman Martin Lee, former "Hong Kong Unity" founding chairman Luo Guancong, and former "Support Association" chairman Lee Cheuk-yan and other chaos elements visited the United States to participate in the "organized by NED" In September 2019, NED recruited anti-China forces to join the board of directors of the Hong Kong Democratic Committee, an anti-China organization headquartered in Washington. The establishment of this organization highlights the symbiotic relationship between anti-China Hong Kong rioters and Washington. Most of its board members are well-known Hong Kong rioters, while the advisory committee of the Hong Kong Democratic Committee is mainly composed of members of non-governmental organizations such as NED. In 2019, during the "case-fixing storm" in Hong Kong, NED arranged for Hong Kong rioters to go to the international community for reactionary propaganda.资助乱港组织活动经费,多次派员赴港指导乱港势力抗争活动。2021年9月,NED举办所谓“未来世界民主发展前景”研讨会,罗冠聪在会上发言,兜售歪理邪说,亵渎正义与真相。“香港民间人权阵线”“香港众志”“香港职工盟”等在“修例风波”中表现突出的组织均获得NED的资助。2021年,NED进一步加大扶植流亡海外“港独”分子群体。
2019年,NED涉港资金约64万美元,具体项目包括:“加强公民社会和人权保护”项目,打“人权”幌子,串联“港独”“民运”团体及政治派领袖向国际社会宣传中央政府“侵犯人权”;“促进循证对话和政策制定”项目,针对香港市民对香港政治、经济问题的看法建立“循证对话”机制,扩大“港独”势力话语权;“扩大工人权利和民主”项目,协助香港工会强化组织、加强谈判和宣传技巧,在香港“推动民主加强公民社会”;“捍卫香港的法治与自由”项目,串联乱港分子和国际商界及政府部门反华势力插手香港法治,炮制研究香港繁荣与法治自由关系的报告。
Five, concoct false information, hype anti-government speech.
1. Distribute inflammatory remarks to provoke people’s anti-government sentiment. In 2021, affected by the COVID-19 epidemic and the tightening of US sanctions, Cuba experienced the worst economic crisis in 30 years. Inflation in Cuba has intensified, and there are shortages of food, medicine and electricity in various places. On July 11th, large-scale anti-government demonstrations broke out in several cities, including the capital Havana. After investigation by the Cuban government, it was found that the demonstration was closely related to American government agencies, in which NED played an important role. A few weeks before the demonstrations broke out, anti-ancient government information on social media began to increase, which effectively manipulated people’s emotions, created dissatisfaction and stimulated protests; A few days before the demonstration, a large number of new account forwarding and unverified anti-ancient government posts suddenly appeared on Twitter, all of which were marked with the label #SOSCuba. The Cuban Foreign Minister said that after investigation, these social media accounts were closely related to a company located in Miami, Florida, USA.
2.炮制涉疆谎言,为遏制中国造势。NED资助的“世界维吾尔代表大会”和“人权观察”组织制造并传播诸如“种族灭绝”“教培中心关押百万维吾尔人”等涉疆谣言。NED支持的“中国人权捍卫者网络”(Chinese Human Rights Defenders,CHRD)仅仅采访8人,便基于这样一个荒谬的小样本“研究”,将估算比例应用到整个新疆,粗暴得出100万人被拘留在“再教育拘留营”,200万人“被迫参加白天或晚上的再教育课程”,炒作涉疆谣言。2019年1月起,美国国务院和NED发起对在美工作学习生活的维吾尔族人进行入户调查。他们询问其家中是否有人在新疆“教培中心”,挑唆其站出来发声“控诉”,煽动针对中国政府的抗议。
3. 传播“政治病毒”,将新冠病毒溯源政治化。疫情暴发后,NED资助的“美国维吾尔人协会”及其下属机构不断传播右翼阴谋论,将疫情和所有相关死亡归咎于中国,并传播中国“正在对世界发动病毒战争”“为了引发大流行故意输出这种病毒”等谣言,为美国和西方国家煽动反华、反亚裔的情绪推波助澜。
4. Create a hostile atmosphere and hype the concept of "sharp strength". In November 2017, Christopher Volcker and jessica Ludwig, deputy directors of the NED Research Department, published in the American Foreign Affairs magazine entitled "The Connotation of Sharp Strength — — How Authoritarian Countries Project their Influence "is the first article to concoct the concept of" sharp power "and advocate a new round of" China threat theory ". In December 2017, NED released the report Sharp Power: Growing Authoritarian Influence, alleging that China and Russia have spent huge sums of money for more than a decade and used "unconventional means such as differentiation, buy-off and manipulation" to influence target countries or groups, so as to influence and shape global public opinion and cognition and demonize China and Russia.
5. Malicious stirring up trouble and stigmatizing China’s media policy. Reporters Without Borders, sponsored by NED, has long encouraged the international community, advertisers, news unions and foreign governments to "treat China’s media differently" and clamored for vigilance against the "threat" of China’s media. After the outbreak of the COVID-19 epidemic, Reporters Without Borders made irresponsible remarks such as "calling for transparency in China’s anti-epidemic information" and "warning the government to increase news restrictions", and created rumors such as "many China journalists are dying in prison".
六、资助活动和学术项目,搞意识形态渗透
1. NED设立所谓“民主”奖项,以鼓励各国异见人士帮助美国“输出民主”。1991年以来,NED每年颁发表彰“捍卫人权和民主”的民主奖(Democracy Award)。该奖项授予俄罗斯、中国、朝鲜、缅甸、伊朗、古巴、委内瑞拉、乌克兰等国的政治活动家和异见人士。1999年以来,NED每年还颁发民主服务奖章(Democracy Service Medal)。2002年,民主服务奖章颁给了时任台湾当局领导人陈水扁的配偶吴淑珍。2010年的奖章颁给了所谓的“西藏流亡精神领袖”第十四世达赖喇嘛。此外,NED还借“世界民主运动”全球大会颁发“民主勇气奖”,颁发对象从第八届(2015年)起出现涉华面孔,此后连续向“藏独”“港独”“东突”等反华组织或个人颁奖,如第八届颁给“港独”分子罗冠聪,第九届(2018年)颁给“维权律师”江天勇家属金变玲,第十届(2021年)颁给英国反华乱港组织“香港监察”、“藏独”组织“自由西藏学生运动”及“东突”组织“维吾尔运动”。其中,罗冠聪为“港独”组织“香港众志”创党主席,因其违法乱港行径被香港警察通缉;江天勇通过蓄意策划“谢阳遭受酷刑”等谣言、插手炒作敏感案件、煽动他人非法聚集滋事及与境外势力勾结等方式,严重威胁国家安全和社会稳定;“香港监察”收到香港警方的警告信,指出该组织涉嫌违反香港国安法第29条“勾结外国或境外势力危害国家安全罪”;“自由西藏学生运动”曾于2008年派出包括该组织执行主席哲东拉珍在内的8名骨干分子潜入中国境内进行破坏活动;“维吾尔运动”为由流亡维吾尔分裂分子组成的极端民族主义组织“世维会”的分支机构,以颠覆中国、建立“东突厥斯坦”民族国家为目标。
2019年6月4日,NED借“八九政治风波”30年,授予“西藏行动中心”“世维会”“对华援助协会”等“藏独”“疆独”“东突”“民运”组织年度“民主奖”。
2. NED has held a series of lectures on Lipsett every year since 2004. The lectures were held in the United States and Canada, and the results of the lectures were published in the journal Democracy. Most of the lecturers are famous political scholars, and the lectures are full of strong ideological colors. For example, in 2020, the lecture was "China under the Dark Shadow of Totalitarianism" by American political scientist Pei Minxin.
3. NED sponsored the non-governmental organization "Egyptian Democratic Institute" to infiltrate ideas in Egypt. In June 2011, Anne Patterson, the new American ambassador to Egypt, admitted that since February 2011, the United States has spent at least $40 million to "promote democracy" in Egypt.
4. In October 2013, the NED core transferee organization "American Democratic Association for International Affairs" received a grant of more than 300,000 US dollars from NED to "improve the communication skills of Venezuelan political activists". Before the local elections in Venezuela in December 2013, the American Democratic Association for International Affairs held a seminar outside Venezuela to provide so-called "expert advice" on how to use technology and social media to "promote citizen outreach and participation". In addition, NED also created a "virtual toolbox" to provide "online customized capacity-building courses on a series of issues related to political innovation", which is still in use today. These measures took effect in the legislative elections in 2015, and the opposition Coalition "Democratic Unity Roundtable" won a historic majority of seats in the Venezuelan National Assembly.
5. 2016年底,NED资助“港独”分子梁天琦、黄台仰分别赴美国哈佛大学和英国牛津大学深造。曾任乱港组织“民间人权阵线”召集人杨政贤2017年参加了该基金会的一个访学项目,“与来自南美、东欧、中东的民间团体领袖和抗争者交流,研究民主运动和社运抗争经验”。
6. NED常年资助举办“族群青年领袖研习班”,参加人员多为“藏独”“疆独”“蒙独”“港独”“台独”及法轮功代表等,截至2020年11月已举办15届。2018年12月,NED时任主席格什曼在第十三届“族群青年领袖研习班”发表主题演讲,称“中国是世界民主最大的威胁”,鼓噪在中国争取“民主”。
7. On June 3, 2019, NED hosted a forum to discuss the topic of "China’s oppression model spread to the world", falsely claiming that the "Chinese oppression model" was "eroding the western democratic system" through a new generation of scientific and technological means.
8. From March 27th to 30th, 2022, NED President Wilson led a delegation to visit Taiwan Province, and held a press conference, announcing that he would cooperate with the Taiwan Democracy Foundation to hold a global conference of the World Democracy Movement in Taipei in October, 2022, in order to support the "Taiwan independence" forces under the guise of "democracy".
9. NED regularly allocates funds to "civil rights" organizations in the name of academic seminars and training. The detailed allocation of NED to Xizang and Xinjiang in 2020 shows that the Foundation provides funds to Tibetan independence and Xinjiang independence organizations such as Xizang Youth Association and World Uighur Congress, and holds seminars to provide forums for exiled Tibetans and Tibetan independence elements in China. Organize the training of Uygur youth’s ability and publicize the "Uighur crisis" in the local community.
10. NED has sponsored political youth in Sudan to participate in training for many years. In 2020, Sudan Regional Center for Civil Society Development and Training (RCDCS) won the "Democracy Award" issued by NED. The organization provides training on "democracy" and radicalism to hundreds of young people all over Sudan.