Pfizer CEO Abel Le data map
What kind of treatment plan will the CEO of "the first pharmaceutical factory in the universe" accept after his nucleic acid test is positive?
On August 15th, local time, official website of Pfizer (NYSE:PFE) issued a statement saying that CEO Albert Bourla tested positive for COVID-19. In a statement, Albright said that she had been vaccinated with four doses of Pfizer/Bionta COVID-19 vaccine, and she felt good but had mild symptoms. She was being quarantined and following public health precautions. In terms of treatment, I have started taking Paxlovid, the company’s oral medicine for COVID-19.
Abel said that he believes that he will recover soon.
Who’s Abel?
On October 1, 2018, Pfizer announced that the board of directors of the company unanimously elected 56-year-old Pfizer COO Abel as CEO of Pfizer, which took effect on January 1, 2019.
According to the official information released by Pfizer China at that time, before becoming COO on January 1, 2018, Abreu led Pfizer’s innovative medical business and established innovative medical emerging market areas. Prior to being appointed as the head of Pfizer’s innovative medical business, Ai Bole became the president of the Vaccine, Oncology and Health Drugs Business Group in January 2014. Under his leadership, the tumor business has tripled in four years, the vaccine business has increased by 50%, and the health medicine business has significantly improved its profitability.
In addition, Arbelot also led several key transactions to promote Pfizer’s investment portfolio in the fields of cancer, inflammation and immunity, vaccines and rare diseases, and to create a strong and leading cancer business in the franchise field of breast cancer and prostate cancer through priority investment.
The history of Pfizer can be traced back to 1849, and it is headquartered in new york, USA. During its development, the forms of acquisition, merger and sale have gradually developed. As the world’s top pharmaceutical company, Pfizer was once called "the first pharmaceutical company in the universe" in the pharmaceutical industry. Under the leadership of Albrecht, Pfizer has made many changes, such as divesting generic drug business in 2020, which was called "the first year of transformation" by Pfizer in 2021, and the company is transforming into a scientific-based, innovative and patient-oriented biopharmaceutical company.
It was also during the period when Albrecht served as CEO that the world experienced the test of COVID-19 epidemic. Pfizer obtained the preventive mRNA COVID-19 vaccine through cooperation with BioNtech, and promoted the use of Paxlovid, a small molecular oral drug in COVID-19, in many markets. These two products also led to the soaring performance of Pfizer. According to the revenue data in 2021, Pfizer ranks second in the world, second only to Johnson & Johnson.
In the second quarter, COVID-19’s vaccine and oral medicine contributed 12.6 billion US dollars in revenue.
In the statement, Aibole mentioned that he had been vaccinated with COVID-19 vaccine and was taking oral medicine, which was also the driving force for Pfizer’s strong performance.
On July 28th, local time, Pfizer released its financial report for the second quarter of 2022, showing that its revenue in the second quarter was US$ 27.7 billion, a year-on-year increase of 47%. In terms of net profit, the revenue in the second quarter was 9.91 billion US dollars, a year-on-year increase of 78%. "In the second quarter, we achieved the largest quarterly sales in history," Pfizer quoted Abel as saying in a press release.
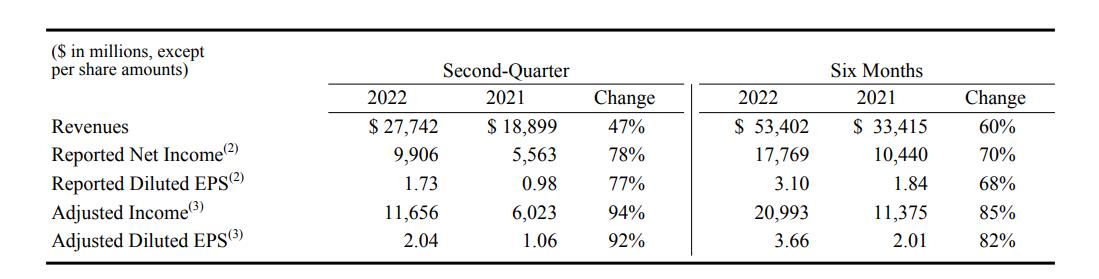
Some data of Pfizer’s second quarterly report
Behind the growth of revenue in the second quarter, it mainly benefited from the contribution of Paxlovid, a new oral drug, and Comirnaty, an mRNA COVID-19 vaccine. Specifically, COVID-19’s sales of oral drugs were $8.1 billion, and COVID-19’s sales of vaccines were $8.8 billion. Together, they contributed $12.6 billion in revenue, accounting for about 45% of the total revenue in the second quarter.
At the end of 2021, the US Food and Drug Administration (FDA) urgently approved Pfizer’s COVID-19 oral drug Paxlovid, which was also the first approved oral COVID-19 drug in the United States. According to the second quarterly report, in the second quarter, the drug was sold in the United States for $4.5 billion, accounting for about 55% of the market sales in the current quarter. According to Pfizer’s first quarterly report this year, the drug contributed $1.47 billion in revenue in the first quarter, with sales in the US market accounting for 69%. Pfizer’s annual sales guideline for the drug remained at $22 billion.
Comirnaty is an mRNA COVID-19 vaccine that Pfizer cooperated with Baiou Entai Company. It was first approved for trial in the United States and other countries and regions at the end of 2020. The second quarter financial report showed that the revenue of the vaccine in the United States was $1.1 billion, down by 47%, while it increased by 43% in other international regions. By July 20th, more than 3.6 billion doses of vaccine had been distributed to 180 countries and regions.
Pfizer expects the annual revenue range to be $98 billion to $102 billion, which is consistent with previous forecasts.